rescuing suboptimal organs
Researchers hope a new technique can treat a variety of damaged organs.
James McCully was in the lab extracting tiny structures called mitochondria from cells when researchers on his team rushed in. They’d been operating on a pig heart and couldn’t get it pumping normally again.
McCully studies heart damage prevention at Boston Children’s Hospital and Harvard Medical School and was keenly interested in mitochondria. These power-producing organelles are particularly important for organs like the heart that have high energy needs. McCully had been wondering whether transplanting healthy mitochondria into injured hearts might help restore their function.
The pig’s heart was graying rapidly, so McCully decided to try it. He loaded a syringe with the extracted mitochondria and injected them directly into the heart. Before his eyes, it began beating normally, returning to its rosy hue.
Since that day almost 20 years ago, McCully and other researchers have replicated that success in pigs and other animals. Human transplantations followed, in babies who suffered complications from heart surgery—sparking a new field of research using mitochondria transplantation to treat damaged organs and disease. In the last five years, a widening array of scientists have begun exploring mitochondria transplantation for heart damage after cardiac arrest, brain damage following stroke, and damage to organs destined for transplantation.
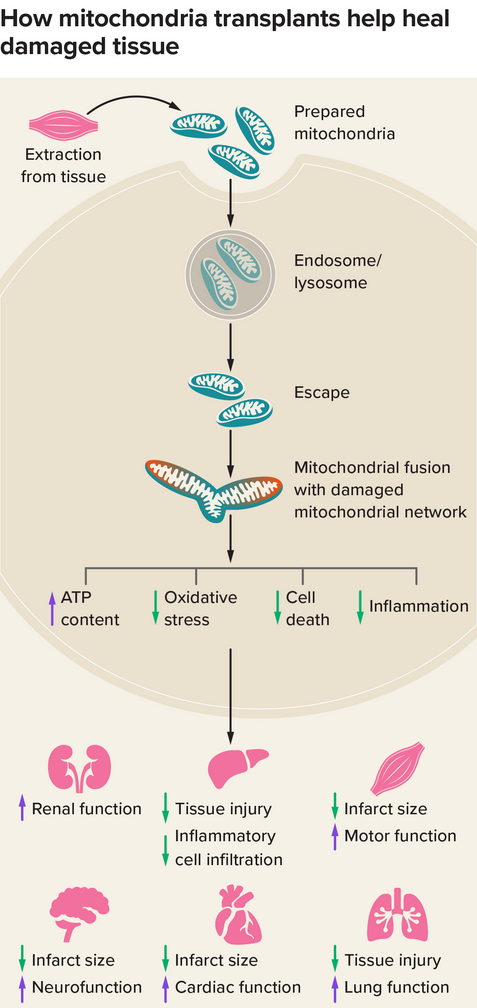
This graphic depicts the basic steps and results of mitochondrial transplantation. Scientists think that donor mitochondria fuse with the recipient cells’ mitochondrial networks. Then they work to shrink the size of the infarct (the area of tissue dying from lack of blood and oxygen), among other effects. Scientists have studied such transplants in kidneys, livers, muscle, brains, hearts, and lungs. Credit: Knowable Magazine
Mitochondria are best known for producing usable energy for cells. But they also send molecular signals that help to keep the body in equilibrium and manage its immune and stress responses. Some types of cells may naturally donate healthy mitochondria to other cells in need, such as brain cells after a stroke, in a process called mitochondria transfer. So the idea that clinicians could boost this process by transplanting mitochondria to reinvigorate injured tissue made sense to some scientists.
From studies in rabbits and rat heart cells, McCully’s group has reported that the plasma membranes of cells engulf the mitochondria and shuttle them inside, where they fuse with the cell’s internal mitochondria. There, they seem to cause molecular changes that help recover heart function: When comparing blood- and oxygen-deprived pig hearts treated with mitochondria to ones receiving placebos, McCully’s group saw differences in gene activity and proteins that indicated less cell death and less inflammation.
About 10 years ago, Sitaram Emani, a cardiac surgeon at Boston Children's Hospital, reached out to McCully about his work with animal hearts. Emani had seen how some babies with heart defects couldn’t fully recover after heart surgery complications and wondered whether McCully’s mitochondria transplantation method could help them.
During surgery to repair heart defects, surgeons use a drug to stop the heart so they can operate. But if the heart is deprived of blood and oxygen for too long, mitochondria start to fail and cells start to die, in a condition called ischemia. When blood begins flowing again, instead of returning the heart to its normal state, it can damage and kill more cells, resulting in ischemia-reperfusion injury.
Since McCully’s eight years of studies in rabbits and pigs hadn’t revealed safety concerns with mitochondria transplantation, McCully and Emani thought it would be worth trying the procedure in babies unlikely to regain enough heart function to come off heart-lung support.
Parents of 10 patients agreed to the experimental procedure, which was approved by the institute’s review board. In a pilot that ran from 2015 to 2018, McCully extracted pencil-eraser-sized muscle samples from the incisions made for the heart surgery, used a filtration technique to isolate mitochondria and checked that they were functional. Then the team injected the organelles into the baby’s heart.
Eight of those 10 babies regained enough heart function to come off life support, compared to just four out of 14 similar cases from 2002 to 2018 that were used for historical comparison, the team reported in 2021. The treatment also shortened recovery time, which averaged two days in the mitochondrial transplant group compared with nine days in the historical control group. Two patients did not survive — in one case, the intervention came after the rest of the baby’s organs began failing, and in another, a lung issue developed four months later. The group has now performed this procedure on 17 babies.
The transplant procedure remains experimental and is not yet practical for wider clinical use, but McCully hopes that it can one day be used to treat kidney, lung, liver, and limb injuries from interrupted blood flow.
The results have inspired other clinicians whose patients suffer from similar ischemia-reperfusion injuries. One is ischemic stroke, in which clots prevent blood from reaching the brain. Doctors can dissolve or physically remove the clots, but they lack a way to protect the brain from reperfusion damage. “You see patients that lose their ability to walk or talk,” says Melanie Walker, an endovascular neurosurgeon at the University of Washington School of Medicine in Seattle. “You just want to do better and there’s just nothing out there.”
Walker came across McCully’s mitochondrial transplant studies 12 years ago and, in reading further, was especially struck by a report on mice from researchers at Massachusetts General Hospital and Harvard Medical School that showed the brain’s support and protection cells—the astrocytes—may transfer some of their mitochondria to stroke-damaged neurons to help them recover. Perhaps, she thought, mitochondria transplantation could help in human stroke cases too.
She spent years working with animal researchers to figure out how to safely deliver mitochondria to the brain. She tested the procedure’s safety in a clinical trial with just four people with ischemic stroke, using a catheter fed through an artery in the neck to manually remove the blockage causing the stroke, then pushing the catheter further along and releasing the mitochondria, which would travel up blood vessels to the brain.
The findings, published in 2024 in the Journal of Cerebral Blood Flow & Metabolism, show that the infused patients suffered no harm; the trial was not designed to test effectiveness. Walker’s group is now recruiting participants to further assess the intervention’s safety. The next step will be to determine whether the mitochondria are getting where they need to be, and functioning. “Until we can show that, I do not believe that we will be able to say that there’s a therapeutic benefit,” Walker says.
Researchers hope that organ donation might also gain from mitochondria transplants. Donor organs like kidneys suffer damage when they lack blood supply for too long, and transplant surgeons may reject kidneys with a higher risk of these injuries.
To test whether mitochondrial transplants can reinvigorate them, transplant surgeon-scientist Giuseppe Orlando of Wake Forest University School of Medicine in Winston-Salem and his colleagues injected mitochondria into four pig kidneys and a control substance into three pig kidneys. In 2023 in the Annals of Surgery, they reported fewer dying cells in the mitochondria-treated kidneys and far less damage. Molecular analyses also showed a boost in energy production.
It’s still early days, Orlando says, but he’s confident that mitochondria transplantation could become a valuable tool in rescuing suboptimal organs for donation.
The studies have garnered both excitement and skepticism. “It’s certainly a very interesting area,” says Koning Shen, a postdoctoral mitochondrial biologist at the University of California, Berkeley, and coauthor of an overview of the signaling roles of mitochondria in the 2022 Annual Review of Cell and Developmental Biology. She adds that scaling up extraction of mitochondria and learning how to store and preserve the isolated organelles are major technical hurdles to making such treatments a larger reality. “That would be amazing if people are getting to that stage,” she says.
“I think there are a lot of thoughtful people looking at this carefully, but I think the big question is, what’s the mechanism?” says Navdeep Chandel, a mitochondria researcher at Northwestern University in Chicago. He doubts that donor mitochondria fix or replace dysfunctional native organelles, but says it’s possible that mitochondria donation triggers stress and immune signals that indirectly benefit damaged tissue.
Whatever the mechanism, some animal studies do suggest that the mitochondria must be functional to impart their benefits. Lance Becker, chair of emergency medicine at Northwell Health in New York who studies the role of mitochondria in cardiac arrest, conducted a study comparing fresh mitochondria, mitochondria that had been frozen then thawed, and a placebo to treat rats following cardiac arrest. The 11 rats receiving fresh, functioning mitochondria had better brain function and a higher rate of survival three days later than the 11 rats receiving a placebo; the non-functional frozen-thawed mitochondria did not impart these benefits.
It will take more research into the mechanisms of mitochondrial therapy, improved mitochondria delivery techniques, larger trials and a body of reported successes before mitochondrial transplants can be FDA-approved and broadly used to treat ischemia-reperfusion injuries, researchers say. The ultimate goal would be to create a universal supply of stored mitochondria — a mitochondria bank, of sorts — that can be tapped for transplantation by a wide variety of health care providers.
“We’re so much at the beginning—we don’t know how it works,” says Becker. “But we know it’s doing something that is mighty darn interesting.”
This article originally appeared in Knowable Magazine, a nonprofit publication dedicated to making scientific knowledge accessible to all. Sign up for Knowable Magazine’s newsletter.
.png)
 8 hours ago
2
8 hours ago
2












 English (US) ·
English (US) ·